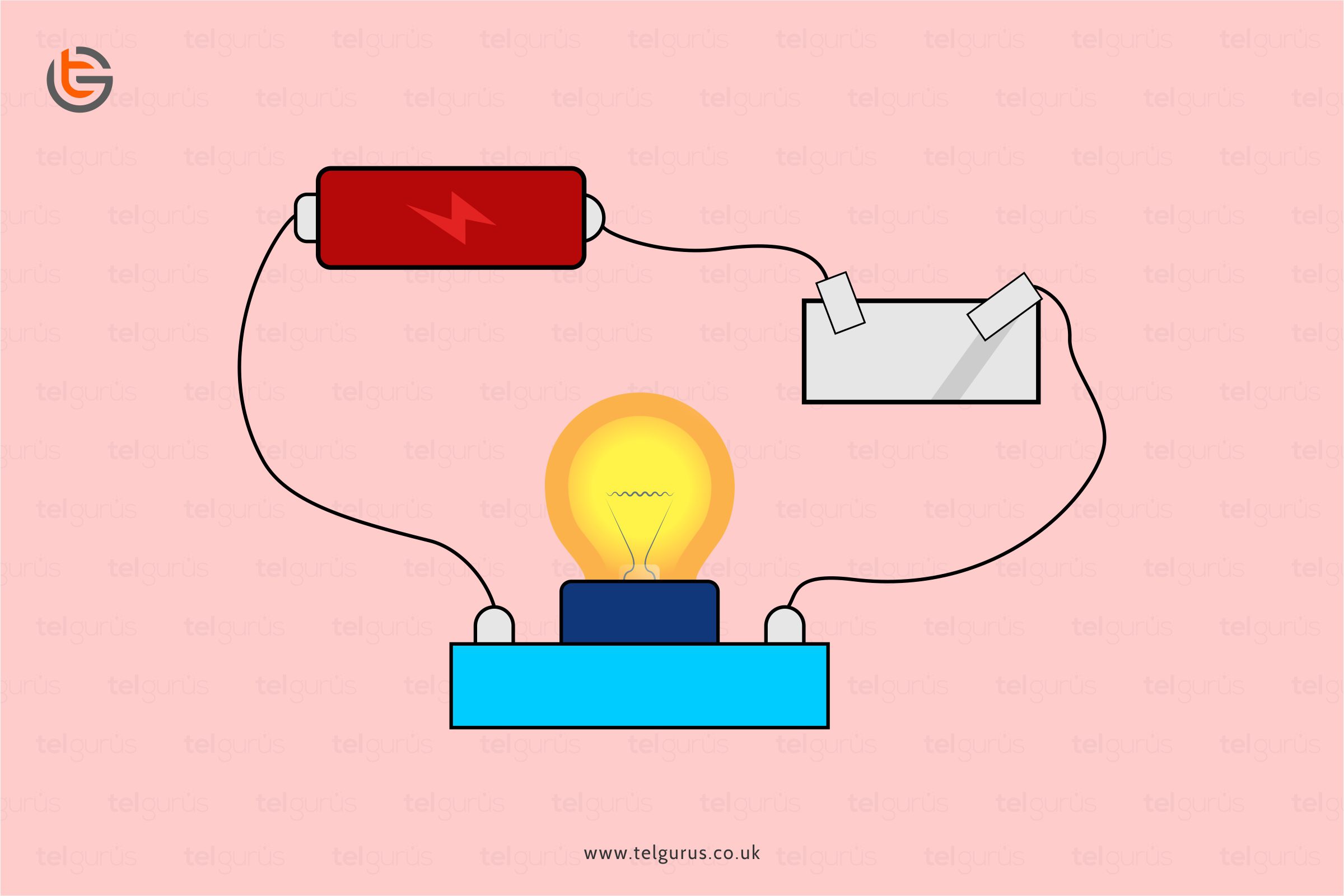
Are Ions A Good Conductor Of Electricity . Web explain the distinction between ionic diffusion and ionic migration. Web this solution is not a good conductor of electric current. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Define the limiting ionic conductivity, and comment. Thus, electrons flow like a “sea of. The larger the concentration of ions, the.
from telgurus.co.uk
The larger the concentration of ions, the. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Define the limiting ionic conductivity, and comment. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web explain the distinction between ionic diffusion and ionic migration. Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Thus, electrons flow like a “sea of. Web this solution is not a good conductor of electric current. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity.
How do metals conduct electricity? Chemistry Questions TEL Gurus
Are Ions A Good Conductor Of Electricity Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. The larger the concentration of ions, the. Define the limiting ionic conductivity, and comment. Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web this solution is not a good conductor of electric current. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web explain the distinction between ionic diffusion and ionic migration. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Thus, electrons flow like a “sea of.
From dokumen.tips
(DOCX) · Web viewUse a word from the box. atoms electrons ions Are Ions A Good Conductor Of Electricity Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Thus, electrons flow like a “sea of. Web this solution is not a good conductor of electric current. Define the limiting ionic conductivity, and comment.. Are Ions A Good Conductor Of Electricity.
From itechguidesss.pages.dev
Does Ice Conduct Electricity itechguides Are Ions A Good Conductor Of Electricity The larger the concentration of ions, the. Thus, electrons flow like a “sea of. Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web metals are good conductors of electricity because they allow. Are Ions A Good Conductor Of Electricity.
From www.youtube.com
What makes Graphite good conductor of electricity ? YouTube Are Ions A Good Conductor Of Electricity Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web the conductor with the comparatively easy. Are Ions A Good Conductor Of Electricity.
From www.slidemake.com
Electrical Conductor Presentation Are Ions A Good Conductor Of Electricity Web explain the distinction between ionic diffusion and ionic migration. The larger the concentration of ions, the. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Thus, electrons flow like a “sea of. Web this solution is not a good conductor of electric current. Define the limiting ionic conductivity, and comment.. Are Ions A Good Conductor Of Electricity.
From sciencenotes.org
What Is the Most Conductive Element? Are Ions A Good Conductor Of Electricity Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web this solution is not a good conductor of electric current. Web explain the distinction between ionic diffusion and ionic migration. The larger the concentration of ions, the. Thus, electrons flow like a “sea of. Define the limiting ionic conductivity, and. Are Ions A Good Conductor Of Electricity.
From atlas-scientific.com
How Do Ions Increase Conductivity? Atlas Scientific Are Ions A Good Conductor Of Electricity Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web this solution is not a good conductor of electric current. Define the limiting ionic conductivity, and comment. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web an electrolyte solution conducts electricity. Are Ions A Good Conductor Of Electricity.
From www.doubtnut.com
In ionic solids, the oppositelycharged ions are closely packed in space Are Ions A Good Conductor Of Electricity Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Thus, electrons flow like a “sea of. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web. Are Ions A Good Conductor Of Electricity.
From www.electricaltechnology.org
Difference Between Conductor, Semiconductor and Insulator Are Ions A Good Conductor Of Electricity Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. The larger the concentration of ions, the. Thus, electrons flow like a “sea of. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web the conductor with the comparatively easy flow of electrons is classified. Are Ions A Good Conductor Of Electricity.
From sciencenotes.org
Examples of Conductors and Insulators Are Ions A Good Conductor Of Electricity Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web explain the distinction between ionic diffusion and ionic migration. Web this solution is not a good conductor of electric current. Define the limiting ionic conductivity, and comment. Web the conductor with the comparatively easy flow of electrons is classified as a good. Are Ions A Good Conductor Of Electricity.
From www.electricity-magnetism.org
What is a conductor? Are Ions A Good Conductor Of Electricity Web this solution is not a good conductor of electric current. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Thus, electrons flow like a “sea of. Web metals are good conductors of electricity. Are Ions A Good Conductor Of Electricity.
From www.youtube.com
Materials that are Good Conductor of Heat and Electricity YouTube Are Ions A Good Conductor Of Electricity The larger the concentration of ions, the. Web explain the distinction between ionic diffusion and ionic migration. Define the limiting ionic conductivity, and comment. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web the. Are Ions A Good Conductor Of Electricity.
From dxoejbyrw.blob.core.windows.net
Why Are Ions Good Conductors Of Electricity at Nellie Ball blog Are Ions A Good Conductor Of Electricity Web explain the distinction between ionic diffusion and ionic migration. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Thus, electrons flow like a “sea of. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. The larger the concentration of ions, the. Web. Are Ions A Good Conductor Of Electricity.
From electricitytohitei.blogspot.com
Electricity Good Conductors Of Electricity Are Ions A Good Conductor Of Electricity Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Thus, electrons flow like a “sea of. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above).. Are Ions A Good Conductor Of Electricity.
From exosepzjf.blob.core.windows.net
Why Is Plastic A Bad Conductor Of Electricity at Tracy Johnson blog Are Ions A Good Conductor Of Electricity Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. Web this solution is not a good conductor of electric current. Thus, electrons flow like a “sea of. Web an electrolyte solution conducts. Are Ions A Good Conductor Of Electricity.
From pubs.rsc.org
New horizons for solid state ion conductors Energy Are Ions A Good Conductor Of Electricity Define the limiting ionic conductivity, and comment. Web explain the distinction between ionic diffusion and ionic migration. Web the conductor with the comparatively easy flow of electrons is classified as a good conductor of electricity. The larger the concentration of ions, the. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of. Are Ions A Good Conductor Of Electricity.
From cityraven.com
😂 Examples of good conductors of heat and electricity. What Are Are Ions A Good Conductor Of Electricity Define the limiting ionic conductivity, and comment. Thus, electrons flow like a “sea of. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). The larger the concentration of ions, the. Web metals are good conductors of electricity because they allow electrons to flow through the entire piece of material. Web explain the. Are Ions A Good Conductor Of Electricity.
From ec.bertabulle.com
How Do Ions Conduct Electricity Are Ions A Good Conductor Of Electricity Define the limiting ionic conductivity, and comment. Web salts that ionize in aqueous solution are great conductors of electricity and are known as electrolytes. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Thus, electrons flow like a “sea of. Web the conductor with the comparatively easy flow of electrons is classified. Are Ions A Good Conductor Of Electricity.
From lessonfullfurmenties.z21.web.core.windows.net
Copper Is A Good Conductor Are Ions A Good Conductor Of Electricity Web explain the distinction between ionic diffusion and ionic migration. Web ionic compounds cannot conduct electricity when solid, as their ions are held in fixed positions and cannot move. Thus, electrons flow like a “sea of. Web an electrolyte solution conducts electricity because of the movement of ions in the solution (see above). Web metals are good conductors of electricity. Are Ions A Good Conductor Of Electricity.